One-stop Service before approval involving Criteria Drafting, Register Tests, Document Organization, Register, Clinical Trial, Progress Tracking.
IVD classification in China
|
Class III: Highest risk
Reagents used in relation to:
1. Test of pathogenic antigen, antibody and nucleic acid;
2. Blood type and tissue typing;
3. Test of human genes;
4. Hereditary diseases;
5. Test of anesthetic, psychiatric drug and toxic medicine for medical purpose;
6. Test of drug target;
7. Test of tumor marker;
8. Allergic reaction (allergen)
Class II: Medium risk
Apart from Class III and Class I products, others fall into Class II products, which mainly include the following reagents detected:
1. Proteins;
2. Glucide;
3. Hormones;
4. Enzymes;
5. Esters;
6. Vitamins;
7. Inorganic ions;
8. Drugs and their metabolic by-products;
9. Autologous Antibody;
10. Microbes Identification (except pathogens) and Drug allergic assays;
11. Other physiological, biochemical and immune function indicators
Class I: Lowest risk
Reagents used in relation to:
1. Microbe Culture medium (excluding for microbe identification and drug allergic assay);
2. Sample treatment, such as hemolytic reagents, dilution solutions and stain solutions.
|
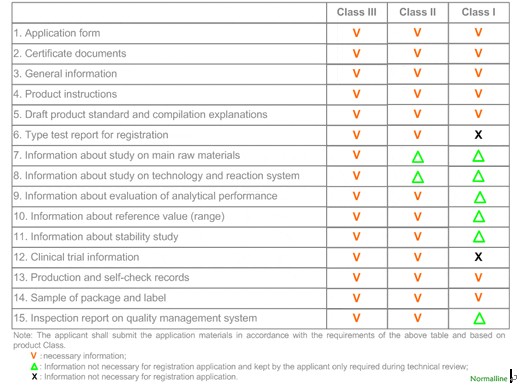
SFDA requirements of the initial registration of IVD reagents
|
The requirements are clarified according to the risk classification of registered IVD reagents.
|
|
|